
This creates the requirement for every FDA or EMA-regulated life science and pharmaceutical manufacturing organisation to validate all computerised systems that can influence the quality of its drug products.įalling under quality systems management (QSM), CSV has had a place in quality and safety compliance for decades.
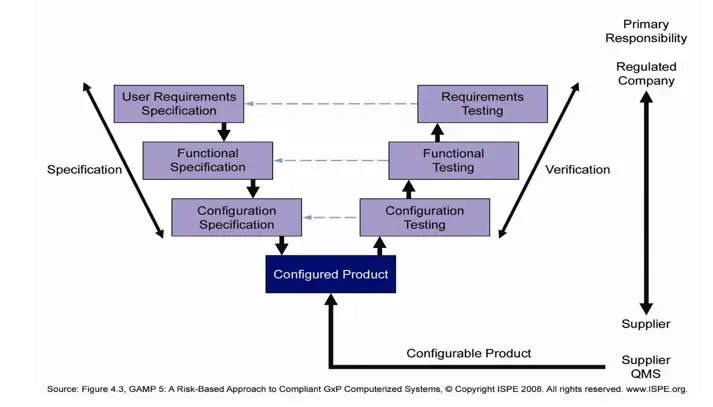
This includes validating computer system capabilities and demonstrating to regulators that system performance and data integrity support current Good Manufacturing Practice (cGMP). Virtually every business and operational aspect involved in making pharmaceuticals, including each link in the global supply chain, is regulated for quality and safety. In this article CSV consultant Brendan Walshe, from Zenith Technologies, a Cognizant Company, frames his perspective on the industry’s appetite for change and outlines how best-practice risk-based approaches can help shift CSV quality systems management into a more efficient and compliant gear. The life sciences industry’s ‘test everything approach’ has become outdated, leaving GMP manufacturing facility operators spending more time documenting than testing. The industry’s increasing dependence on computer-based digital systems requires capital investment and comes with an operational expense that includes the cost of computer system validation (CSV) and compliance-typically estimated to be 25% of overall project costs.

It is a competitive imperative the life sciences industry can’t ignore as it seeks lower-cost manufacturing and faster development timelines to more breakthrough drugs and better patient health outcomes. Pharmaceutical Manufacturing’s fifth annual Smart Pharma survey found just over 88% of pharma manufacturers believe their company will choose to automate processes if given the option and approximately 90% of those responding had begun their digital transformation efforts1.


From the earliest clinical phases to the last post-approval study, the data and technical documentation these systems manage are essential to the safe, effective creation of medicines and therapeutics for all patients.Įvery passing year, the life sciences industry’s approach to continuous improvement has seen the increasing application of computerised systems to deliver better results. At the heart of almost all life science and pharmaceutical industry operations are digital information and document handling systems.


 0 kommentar(er)
0 kommentar(er)
